MAKE A MEME
View Large Image
| View Original: | Origins.gif (450x303) | |||
| Download: | Original | Medium | Small | Thumb |
| Courtesy of: | commons.wikimedia.org | More Like This | ||
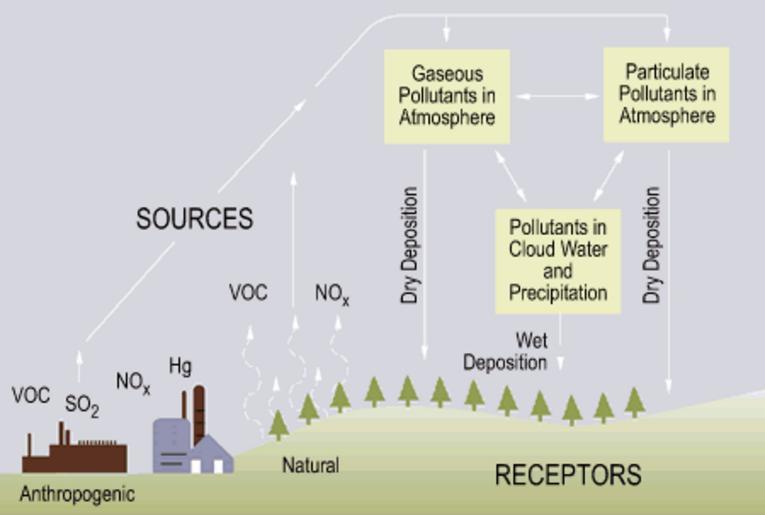
| Keywords: Origins.gif Origins_of_acid_rain svg Flow chart showing dry and wet deposition processes <br>Original site Text <br> Power plant emitting substances into the air Acid rain is a broad term referring to a mixture of wet and dry deposition deposited material from the atmosphere containing higher than normal amounts of nitric and sulfuric acids The precursors or chemical forerunners of acid rain formation result from both natural sources such as volcanoes and decaying vegetation and man-made sources primarily emissions of sulfur dioxide SO2 and nitrogen oxides NOx resulting from fossil fuel combustion In the United States roughly 2/3 of all SO2 and 1/4 of all NOx come from electric power generation that relies on burning fossil fuels like coal Acid rain occurs when these gases react in the atmosphere with water oxygen and other chemicals to form various acidic compounds The result is a mild solution of sulfuric acid and nitric acid When sulfur dioxide and nitrogen oxides are released from power plants and other sources prevailing winds blow these compounds across state and national borders sometimes over hundreds of miles Flow chart showing dry and wet deposition processes If you have difficulty viewing this graphic or need additional information contact Cindy Walke Web Manager at 202-343-9194 Wet Deposition Wet deposition refers to acidic rain fog and snow If the acid chemicals in the air are blown into areas where the weather is wet the acids can fall to the ground in the form of rain snow fog or mist As this acidic water flows over and through the ground it affects a variety of plants and animals The strength of the effects depends on several factors including how acidic the water is; the chemistry and buffering capacity of the soils involved; and the types of fish trees and other living things that rely on the water Dry Deposition In areas where the weather is dry the acid chemicals may become incorporated into dust or smoke and fall to the ground through dry deposition sticking to the ground buildings homes cars and trees Dry deposited gases and particles can be washed from these surfaces by rainstorms leading to increased runoff This runoff water makes the resulting mixture more acidic About half of the acidity in the atmosphere falls back to earth through dry deposition http //www3 epa gov/acidrain/what/ >http //www epa gov/acidrain/images/origins gif gif 2012-11-05 servertimestamp n/a PD-USGov original upload log page en wikipedia Origins gif 2006-02-12 16 41 NHSavage 450×303× 13412 bytes <nowiki>Downloaded from US EPA website http //www epa gov/acidrain/origins gif</nowiki> Acid rain | ||||