MAKE A MEME
View Large Image
| View Original: | Rutherford gold foil experiment results.svg (301x460) | |||
| Download: | Original | Medium | Small | Thumb |
| Courtesy of: | commons.wikimedia.org | More Like This | ||
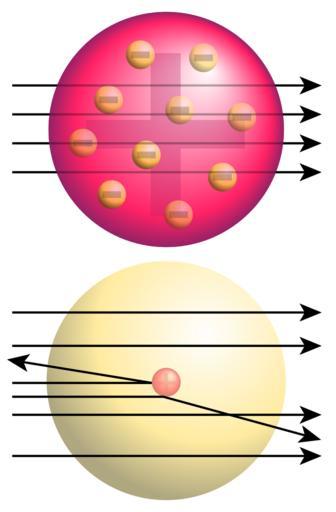
| Keywords: Rutherford gold foil experiment results.svg Top Expected results of Rutherford's gold foil experiment alpha particles passing through the plum pudding model of the atom undisturbed Bottom Observed results Some of the particles were deflected and some by very large angles Rutherford concluded that the positive charge of the atom must be concentrated into a very small location the atomic nucleus Own 2008-04 Drawn by User Fastfission in Illustrator and Inkscape --Fastfission 15 04 14 April 2008 UTC If you want to credit someone credit Wikimedia Commons Otherwise don't credit anyone that's fine by me Fastfission Rutherford scattering Models of atoms | ||||